The following Latimer diagrams show the standard reduction potentials E /V for some oxidation states of
Question:
The following Latimer diagrams show the standard reduction potentials E⦵/V for some oxidation states of iron in acid and alkaline solution:
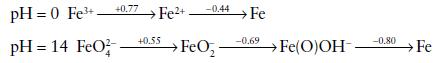
(a) Plot a Frost diagram showing the states of Fe under acid and alkaline conditions.
(b) Calculate the standard potential for the Fe3+/Fe couple in acid
solution.
(c) Explain why the potentials for the Fe(III)/Fe(II) reduction are very different in acid and alkaline conditions, and comment on the fact that FeO42− is unknown in acid solution.
(d) FeO42− can be prepared by oxidation of Fe(III) by ClO− in alkaline solution. Making use of the standard reduction potentials given below, write an equation for the reaction occurring in this preparation.
Standard potentials E⦵/V at pH = 14:
![]()
Step by Step Answer:

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke





