The glass industry manufactures millions of tonnes of glass per year. (a) Only certain element oxides form
Question:
The glass industry manufactures millions of tonnes of glass per year.
(a) Only certain element oxides form glasses. Explain why this is, giving examples of what are termed in the glass industry as ‘networkforming oxides’. Which oxide is the most important starting material in commercial glasses? Explain how a glass differs from a crystalline oxide such as α-Al2O3 (corundum).
(b) Glass can be ‘modified’ by adding oxides such as Na2O or CaO. Suggest how an O2− ion might interact with the original oxide network. What role will the Na+ or Ca2+ ions play in the modified glass?
(c) A modified glass is treated with Al2O3 and the reaction is represented by the following equation:
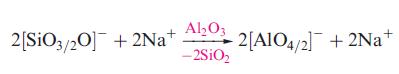
Explain the meaning of the fractional notation used in the equation and show schematically how the structure of the glass is altered when Al2O3 is added.
Step by Step Answer:






