As a chemical engineer working for MedAid, Inc., you are assigned to size a reactor for producing
Question:
As a chemical engineer working for MedAid, Inc., you are assigned to size a reactor for producing Agent X, a new chemical designed to combat killer viruses. X is produced by the following liquid-phase reaction:
A + Z →X where the reaction rate is first-order in species A and first-order in species Z (second-order overall) and is described by
![]()
The activation energy for the reaction is 31.5 kcal/gmol, and ko = 2.0 × 1016 L/gmol s. Species A (MW = 102) and species Z (MW = 76) are to enter the reactor at a rate of 22 gmol/s and 27 gmol/s, respectively. An additional inert (non-reacting) component, species I (MW = 25), also enters the reactor at a rate of 38 gmol/s. The desired conversion of A in the reactor is 80%. A single product stream leaves the reactor and has a density (as a function of temperature) that is described by
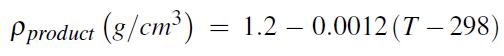
where T is the temperature in units of K.
a. Use a spreadsheet to calculate the necessary reactor volume as a function of temperature for a range of temperatures from 298 K to 375 K. The calculation should be performed for at least 15 temperatures in this range. Your answer should include a table (printed from the spreadsheet) that provides values of kr, ρproduct , V̇product , rreaction,A, and Vreactor (in liters) as a function of temperature. Note that the molar and mass flow rates are not a function of temperature.
b. Does the required reactor volume increase with increasing temperature, or decrease? Why?
c. At what temperature (within the specified range) would you recommend operating the reactor? Why? What is the required reactor volume at this temperature?
d. Is it feasible to operate the reactor at room temperature? Why, or why not?
e. What practical or physical considerations not included in the calculations might limit or influence the practical operating temperature of the reactor? (List at least two.)
Step by Step Answer:

Introduction To Chemical Engineering Tools For Today And Tomorrow
ISBN: 9780470885727
5th Edition
Authors: Kenneth A. Solen, John N. Harb





