The exhaust gas coming from a coal-burning furnace (flue gas) usually contains sulfur in the form of
Question:
The exhaust gas coming from a coal-burning furnace (flue gas) usually contains sulfur in the form of SO2, and when the gas is discharged into the atmosphere (which sometimes happens), the SO2 can eventually react with oxygen and water to form sulfuric acid (H2SO4), hence, acid rain. The reaction is
![]()
The air around an old power plant has the following average composition:
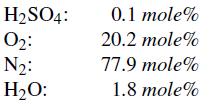
What is the number of grams of sulfuric acid per ton (2000 lbm) of this air?
Transcribed Image Text:
SO₂ + O2 + H₂O → H₂SO4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
H2SO4 01 O2 202 N2 779 H2O 18 Average Molecular weight of this air X H2SO4 M H2SO4 X O2 M O2 X ...View the full answer

Answered By

Atuga Nichasius
I am a Highly skilled Online Tutor has a Bachelor’s Degree in Engineering as well as seven years of experience tutoring students in high school, bachelors and post graduate levels. I have a solid understanding of all learning styles as well as using asynchronous online platforms for tutoring needs. I individualise tutoring for students according to content tutoring needs assessments.
My strengths include good understanding of all teaching methods and learning styles and I am able to convey material to students in an easy to understand manner. I can also assists students with homework questions and test preparation strategies and I am able to help students in math, gre, business , and statistics
I consider myself to have excellent interpersonal and assessment skills with strong teaching presentation verbal and written communication
I love tutoring. I love doing it. I find it intrinsically satisfying to see the light come on in a student's eyes.
My first math lesson that I taught was when I was 5. My neighbor, still in diapers, kept skipping 4 when counting from 1 to 10. I worked with him until he could get all 10 numbers in a row, and match them up with his fingers.
My students drastically improve under my tutelage, generally seeing a two grade level improvement (F to C, C to A, for example), and all of them get a much clearer understanding!
I am committed to helping my students get the top grades no matter the cost. I will take extra hours with you, repeat myself a thousand times if I have to and guide you to the best of my ability until you understand the concept that I'm teaching you.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Tools For Today And Tomorrow
ISBN: 9780470885727
5th Edition
Authors: Kenneth A. Solen, John N. Harb
Question Posted:
Students also viewed these Engineering questions
-
Sulfuric acid is the chemical produced in the United States with the highest volume of production. In one of the earliest processes used to make it, an ore containing iron pyrites (FeS 2 ) is roasted...
-
The quantity of sulfuric acid used globally places it among the most plentiful of all commodity chemicals. In the modern chemical industry, synthesis of most sulfuric acid utilizes elemental sulfur...
-
A Claus plant converts gaseous sulfur compounds to elemental sulfur, thereby eliminating emission of sulfur into the atmosphere. The process can be especially important in the gasification of coal,...
-
When Steve Jobs first demonstrated "the pinch"-the two-finger gesture to zoom in and out on photos and Web pages on the iPhone, it just rocked the mobile phone industry-the whole digital world heard...
-
Suppose the economy is in a long-run equilibrium. a. Draw the economys short-run and long-run Phillips curves. b. Suppose a wave of business pessimism reduces aggregate demand. Show the effect of...
-
The demand curve for ice cream in a small town has been stable for the past few years. In most months, when the equilibrium price is $3 per serving for the most popular ice cream, customers buy 300...
-
Let \(D\) denote the event that you have the illness, and let \(S\) denote the event that the test signals positive. The probability requested can be denoted as \(P(D \mid S)\). The probability that...
-
Prepare a performance report for Imperial Data Devices using the budget information from Exercise 10-15 and the next performance information. In Exercise 10-15, Expected manufacturing costs for...
-
Please help me as much as you can!! I will take all your effort to solve this problem and will give you a good rate!!! Please show all the calculations in detail! Also, please do not copied and...
-
In this chapter, we mention that problems arise from having at least two systems of units in the industrialized world (i.e., the metric system and the American engineering system). In more specific...
-
Name three examples of heat exchangers that are part of your everyday life.
-
Alice invested $100 per month in an RRSP for 20 years. Her sister invested $1200 at the end of each year, also for 20 years. Suppose that both RRSP accounts can earn 4.5% compounded annually. How...
-
What kinds of conflict could erupt during the storming phase of team development? Should conflict be avoided? Explain.
-
Discuss five ways to achieve the best results during a virtual meeting.
-
What is a buried verb, and how can you avoid these in your writing?
-
Suppose for the previous Problem 3.21, that your firm has decided to raise their required rate of return to 20%. How long will it take to pay back your initial investment now? Problem 3.21 Discounted...
-
List three ground rules for meetings. As part of your answer, discuss why it is important to establish these ground rules before the meeting.
-
Prepare journal entries to record the following transactions involving the short-term securities investments of Bolton Co., all of which occurred during year 2011. a. On February 15, paid $170,000...
-
1) The government decided to reduce taxes on fast-food to increase revenue. The government assumes that fast-food products have a) An inelastic demand b) An elastic demand c) A demand curve that is...
-
An investor wants a real rate of return if (rate of return without inflation) of 10% per year on any projects in which he invests. If the expected annual inflation rate for the next several years is...
-
(a) Compute the equivalent annual inflation rate, based on the consumer price index, for the period from 1981 to' 1986. (b) Using the equivalent annual inflation rate computed in part (a), estimate...
-
You are considering the purchase, for $15,000, of an annuity that pays $2500 per year for the next 10 years. You want to have a real rate of return of 5%, and you estimate inflation will average 6%...
-
When you retire you would like to have saved enough to draw out cash each year starting on your 65th birthday. Today is your 20th birthday. The first withdrawal will be $50,000 and each withdrawal...
-
Consider the securities shown here that are trading at their respective market prices. The two securities pay risk-free cash flows over the next two years. Security A Security B Market Prices CF in...
-
Lance Whittingham IV specializes in buying deep discount bonds. These represent bonds that are trading at well below par value. He has his eye on a bond issued by the Leisure Time Corporation. The...

Study smarter with the SolutionInn App


