A rigid container is filled with liquid acetone at 20C and 1 bar. Through heat transfer at
Question:
A rigid container is filled with liquid acetone at 20°C and 1 bar. Through heat transfer at constant volume, a pressure of 100 bar is generated. CP = 125 J/mol-K. (Other properties of acetone are given in problem 6.10.) Provide your best estimate of the following:
(a) The temperature rise
(b) ΔS, ΔU, and ΔH
(c) The heat transferred per mole
Data from problem 6.10:
Determine the difference CP - CV for the following liquids using the data provided near 20°C.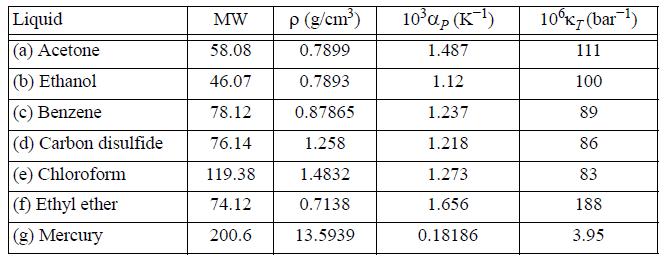

Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





