Consider a closed vessel initially containing 1 mol of tetrahydrofuran at 50C and 52 kPa. Imagine that
Question:
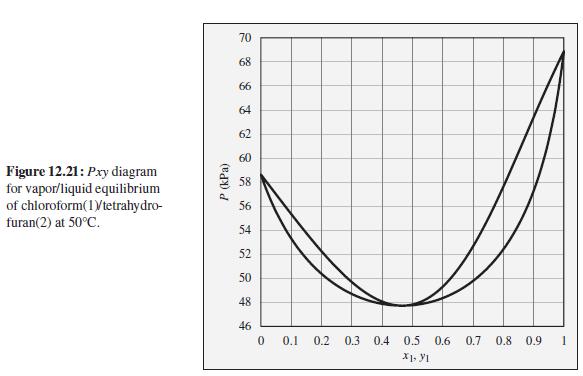
Consider a closed vessel initially containing 1 mol of tetrahydrofuran at 50°C and 52 kPa. Imagine that pure chloroform is slowly added at constant temperature and pressure until the vessel contains 1 mol tetrahydrofuran and 9 mol chloroform. Describe the evolution of phases and phase compositions during this process. Comment on the practical feasibility of carrying out such a process. What sort of device would be required? How would the total system volume change during this process? At what composition would the system volume reach its maximum value?
To the Pxy diagram for chloroform(1)/tetrahydrofuran(2) at 50°C shown in Fig. 12.21.
Step by Step Answer:

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart





