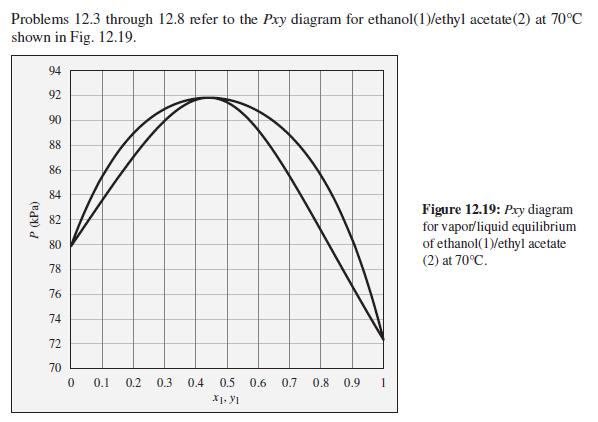
Consider a closed vessel initially containing 1 mol of pure ethyl acetate at 70C and 86 kPa.
Question:
Consider a closed vessel initially containing 1 mol of pure ethyl acetate at 70°C and 86 kPa. Imagine that pure ethanol is slowly added at constant temperature and pressure until the vessel contains 1 mol ethyl acetate and 9 mol ethanol. Describe the evolution of phases and phase compositions during this process. Comment on the practical feasibility of carrying out such a process. What sort of device would be required? How would the total system volume change during this process? At what composition would the system volume reach its maximum value?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:





