Even in the days of van der Waals, the second virial coefficient for square-well fluids ( =
Question:
Even in the days of van der Waals, the second virial coefficient for square-well fluids (λ = 1.5) was known to be B/b = 4 + 9.5 [exp(NAε/RT) -1]. Noting that ex ~ 1 + x + x2/2 + …, this observation suggests the following equation of state: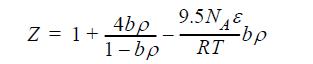
Derive an expression for the Helmholtz energy departure function for this equation of state.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





