Five grams of the specified pure solvent is placed in a variable volume piston. What is the
Question:
Five grams of the specified pure solvent is placed in a variable volume piston. What is the volume of the pure system when 50% and 75% have been evaporated at:
(i) 30°C,
(ii) 50°C?
Use the Antoine equation (Appendix E) to relate the saturation temperature and saturation pressure. Use the ideal gas law to model the vapor phase. Show that the volume of the system occupied by liquid is negligible compared to the volume occupied by vapor.
(a) Hexane (ρL = 0.66 g/cm3)
(b) Benzene (ρL = 0.88 g/cm3)
(c) Ethanol (ρL = 0.79 g/cm3)
(d) Water without using the steam tables (ρL = 1 g/cm3)
(e) Water using the steam tables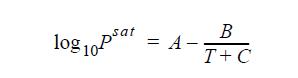
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





