For T r r P r sat , the Peng-Robinson equation of state has three roots
Question:
For Tr r ≈ Prsat, the Peng-Robinson equation of state has three roots corresponding to compressibility factors between zero and 10. The smallest root is the compressibility factor of the liquid. The largest root is the compressibility factor of the vapor and the middle root has no physical significance. This gives us a general method for finding the compressibility factor of any fluid obeying the Peng-Robinson equation. For the iterative method, use an initial guess of Z = 0 to find the liquid roots and Z = 1 to find the vapor roots of methane at the following conditions: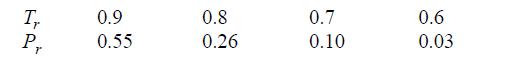
Compare to experimental data from N.B. Vargaftik. 1975. Handbook of Physical Properties of Liquids and Gases, 2nd ed., New York: Hemisphere.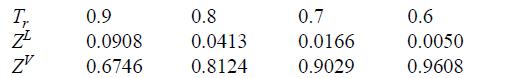
Step by Step Answer:

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira





