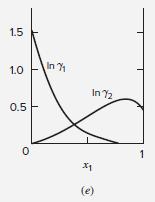
For the ethanol(1)/chloroform(2) system at 50C, the activity coefficients show interior extrema with respect to composition [see
Question:
For the ethanol(1)/chloroform(2) system at 50°C, the activity coefficients show interior extrema with respect to composition [see Fig. 13.4(e)].
(a) Prove that the van Laar equation cannot represent such behavior.
(b) The two-parameter Margules equation can represent this behavior, but only for particular ranges of the ratio A21∕A12. What are they?
Figure 13.4 (e)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:





