If U is considered a function of T and P, the natural heat capacity is neither C
Question:
If U is considered a function of T and P, the “natural” heat capacity is neither CV nor CP, but rather the derivative (∂U/∂T)P . Develop the following connections between (∂U/∂T)P , CP, and CV:
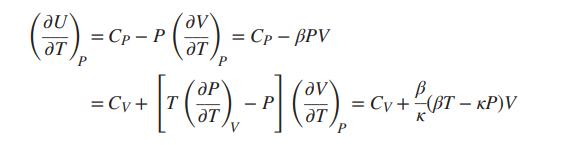
To what do these equations reduce for an ideal gas? For an incompressible liquid?
Transcribed Image Text:
(27) = C₂-P (27) = C₂ P =Cp-BPV ++ [* (*), -*] (@x), -cv+fur - t · T кP)V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The equations you provided seem to involve a mix of symbols and notation that are not standard in th...View the full answer

Answered By

Abdul Wahab Qaiser
Before working at Mariakani, I volunteered at a local community center, where I tutored students from diverse backgrounds. I helped them improve their academic performance and develop self-esteem and confidence. I used creative teaching methods, such as role-playing and group discussions, to make the learning experience more engaging and enjoyable.
In addition, I have conducted workshops and training sessions for educators and mental health professionals on various topics related to counseling and psychology. I have presented research papers at conferences and published articles in academic journals.
Overall, I am passionate about sharing my knowledge and helping others achieve their goals. I believe that tutoring is an excellent way to make a positive impact on people's lives, and I am committed to providing high-quality, personalized instruction to my students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781260597684
9th International Edition
Authors: J.M. Smith, Mark Swihart Hendrick C. Van Ness, Michael Abbott
Question Posted:
Students also viewed these Engineering questions
-
The electric and the magnetic field associated with an E.M. wave, propagating along the +z-axis, can be represented by ) [E = Eo, B = Boj] (b) [E = Eok, B = Bo] (c) [E = Eo, B = Bo] (d) [E = E), B =...
-
For a Van der Waals gas find: (a) The equation of the adiabatic curve in the variables T, V; (b) The difference of the molar heat capacities Cp = Cv as a function of T and V.
-
The specific heat at constant pressure for an ideal gas is given by cp = 0.9 + (2.7 x 10-4) T (kJ/kg K) where T is in kelvin. The change in the enthalpy for this ideal gas undergoing a process in...
-
Sumit's age after 12 years will be 6 times his age 8 years back. What is the present age of Sumit? (a) 10 (b) 12 (c) 14 (d) 15 (e) 18
-
Suppose that on the basis of the analysts past record, you estimate that the relationship between forecast and actual alpha is: Actual abnormal return = .3 Forecast of alpha Use the alphas from...
-
The manager of a fish market pays 80 cents each for cod and sells them for $1.50 each. Fish left over at the end of the day are discarded. The daily demand can be approximated by a normal...
-
Using the gasoline mileage data in Table B. 3 find the eigenvectors associated with the smallest eigenvalues of \(\mathbf{X}^{\prime} \mathbf{X}\). Interpret the elements of these vectors. What can...
-
Your company has two divisions: One division sells software and the other division sells computers through a direct sales channel, primarily taking orders over the Internet. You have decided that...
-
Why do investors allocate capital across asset classes? Referring to the Financial Analysts Journal article, is the recent shift into low-risk, cash-like assets by many fund managers a type of market...
-
Bobby Reynolds, a new client of yours, is a selfemployed caterer in Santa Fe, New Mexico. Bobby drives his personal van when delivering catered meals to customers. You have asked him to provide the...
-
A steam plant operates on the cycle of Fig. 8.4. The pressure levels are 10 kPa and 6000 kPa, and steam leaves the turbine as saturated vapor. The pump efficiency is 0.70, and the turbine efficiency...
-
Anglo American plc is a multinational mining company with its headquarters in South Africa. It is the worlds largest producer of platinum as well as a major producer of diamond, copper, nickel, and...
-
For the following exercises, eliminate the parameter t to rewrite the parametric equation as a Cartesian equation. x(t) = -t |y(t) = t + 1
-
Define human resource audit. State its advantages and limitations. How does it differ from human resource accounting?
-
What are the contents and format of an audit report?
-
Can dividend be paid out of profit arising out of forfeited and re-issue of shares?
-
State with reasons whether you, as an auditor, would approve the payment of dividend out of capital.
-
How the auditors prepare themselves before undertaking audit of an insurance company?
-
Between 1964 and 1966, Ford made more than 1 million Mustangs. Today car collectors are spending tens of thousands of dollars to restore to "like new" vintage Mustangs that originally sold for around...
-
A firm offers two products for sale. The marginal cost of one product is new zero once the first unit has been produced. The marginal cost of the other product rises as output rises. What would be...
-
The actone(1) + chloroform(2) system has an azeotrope at x 1 = 0.38, 248 mmHg, and 35.17C. Fit the Wilson equation, and predict the P-x-y diagram.
-
Show that Wilsons equation reduces to Florys equation when A ij = A ji = 0. Further, show that it reduces to an ideal solution if the energy parameters are zero, and the molecules are the same size.
-
As part of a biorefining effort, butanediols are being produced by fermentation. The problem is that the isomers are all mixed up. Furthermore, 1,3-propanediol comprises roughly 30mol% of the mixture...
-
1. Market liquidity. What happens to a firm's marginal cost of capital as it expands in an illiquid market? How can it overcome these difficulties? 2. Barriers to Cross-Listing. What are the main...
-
Mark Hancock Incorporated manufactures a specialized surgical Instrument called the HAN-20. The firm has grown rapidly in recent years because of the product's low price and high quality. However,...
-
Exercise 21-3 (Algo) Preparing flexible budgets LO P1 Tempo Company's fixed budget (based on sales of 16,000 units) folllows. Fixed Budget Direct labor Sales (16,000 units $208 per unit) Costs Direct...

Study smarter with the SolutionInn App


