Make use of Eqs. (3.36), (3.61), (3.62), (6.54), (6.55), (6.56), (6.70), (6.71), (10.62), and (10.69)(10.74), to estimate
Question:
Make use of Eqs. (3.36), (3.61), (3.62), (6.54), (6.55), (6.56), (6.70), (6.71), (10.62), and (10.69)–(10.74), to estimate V, HR, SR, and GR for one of the following binary vapor mixtures:
(a) Acetone(1)/1,3-butadiene(2) with mole fractions y1 = 0.28 and y2 = 0.72 at t = 60°C and P = 170 kPa.
(b) Acetonitrile(1)/diethyl ether(2) with mole fractions y1 = 0.37 and y2 = 0.63 at t = 50°C and P = 120 kPa.
(c) Methyl chloride(1)/ethyl chloride(2) with mole fractions y1 = 0.45 and y2 = 0.55 at t = 25°C and P = 100 kPa.
(d) Nitrogen(1)/ammonia(2) with mole fractions y1 = 0.83 and y2 = 0.17 at t = 20°C and P = 300 kPa.
(e) Sulfur dioxide(1)/ethylene(2) with mole fractions y1 = 0.32 and y2 = 0.68 at t = 25°C and P = 420 kPa.
Eq. (3.36)

Eq. (3.61)
![]()
Eq. (3.62)
![]()
Eq. (6.54)

Eq. (6.55) & (6.56)
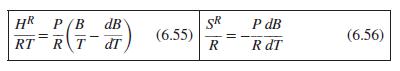
Eq. (6.70) & (6.71)
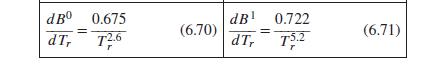
Eq. (10.62)
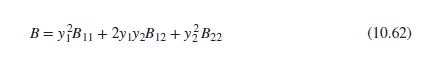
Eq. (10.69)

Eq. (10.74)
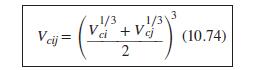
Step by Step Answer:

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart





