The ESD equation is presented in problem 7.19. Derive expressions for the enthalpy and entropy departure functions
Question:
The ESD equation is presented in problem 7.19. Derive expressions for the enthalpy and entropy departure functions in terms of this equation of state.
Data from Problem 7.19
The ESD equation of state is given by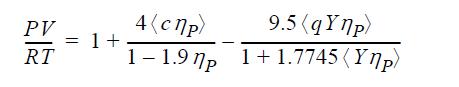
ηP = bρ, c is a “shape parameter” which represents the effect of non-sphericity on the repulsive term, and q = 1 + 1.90476(c - 1). A value of c = 1 corresponds to a spherical molecule. Y is a temperature-dependent function whose role is similar to the temperature dependence of the a parameter in the Peng-Robinson equation. Use the methods of Example 7.7 to fit b and Y to the critical point for ethylene using c = 1.3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





