The human body processes ethanol by oxidizing it to acetaldehyde via the NAD ox/ NAD red dehydrogenase
Question:
The human body processes ethanol by oxidizing it to acetaldehyde via the NADox/NADred dehydrogenase redox reaction. The reaction is![]()
The values for properties in the order the species appear in the reaction are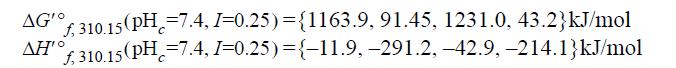
(a) Determine the magnitude of the heat of reaction under the stated conditions.
(b) Calculate the equilibrium constant. If we assume the ratio of the two forms of NAD are near unity, what is implied about the ratio of acetaldehyde:ethanol? What is the importance of sign of the Gibbs energy for the subsequent oxidation of acetaldehyde to acetic acid?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





