The following is a set of VLE data for the system acetone(1)/methanol(2) at 55C: (a) Basing calculations
Question:
The following is a set of VLE data for the system acetone(1)/methanol(2) at 55°C:
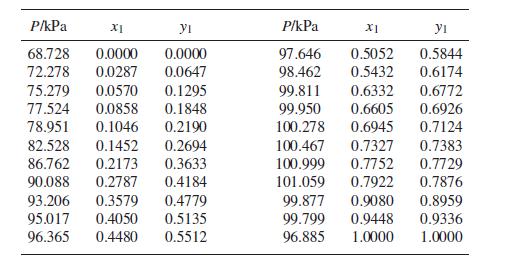
(a) Basing calculations on Eq. (13.24), find parameter values for the Margules equation that provide the best fit of GE∕RT to the data, and prepare a Pxy diagram that compares the experimental points with curves determined from the correlation.
(b) Repeat (a) for the van Laar equation.
(c) Repeat (a) for the Wilson equation.
(d) Using Barker’s method, find parameter values for the Margules equation that provide the best fit of the P–x1 data. Prepare a diagram showing the residuals δP and δy1 plotted vs. x1.
(e) Repeat (d) for the van Laar equation.
(f) Repeat (d) for the Wilson equation.
Eq (13.24)
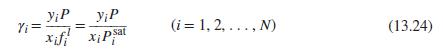
Step by Step Answer:

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart





