Water + hexane and water + benzene are immiscible pairs. (a) The binary system water + benzene
Question:
Water + hexane and water + benzene are immiscible pairs.
(a) The binary system water + benzene boils at 69.4 °C and 760 mmHg. What is the activity coefficient of benzene in water if the solubility at this point is xB = 1.6E-4, using only this information and the Antoine coefficients?
(b) What is the vapor composition at the bubble pressure at room temperature (292 K) for a ternary mixture consisting of 1 mole overall of each component if the organic layer is assumed to be an ideal solution?
(c) What is the vapor composition at the bubble pressure at room temperature (292 K) for a ternary mixture consisting of 1 mole overall of each component if the activity coefficients of the organic layer are predicted by UNIFAC?
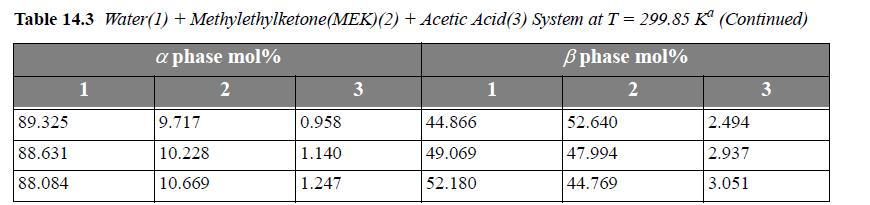
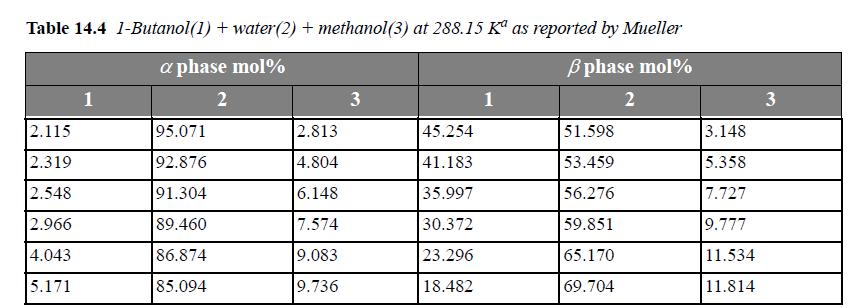
The following problems concern LLE in ternary systems. Experimental data for the systems are listed in Tables 14.3 and 14.4.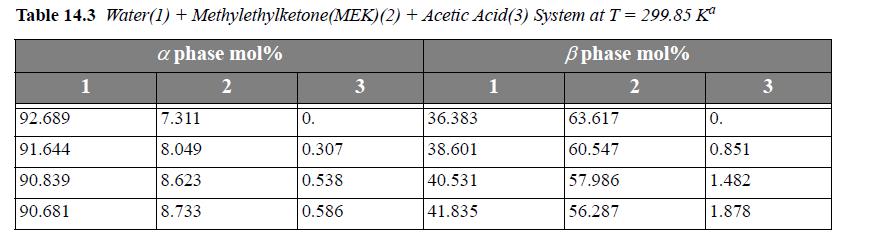
Step by Step Answer:

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira





