A group of students came across an unsuspected supply of laboratory alcohol containing 96 weight % ethanol
Question:
A group of students came across an unsuspected supply of laboratory alcohol containing 96 weight % ethanol (EtOH) and 4 weight % water (H2O) . As an experiment, they wanted to see if they could make exactly 2.00 L of vodka having a composition of 56 weight % ethanol. Determine the volume of lab alcohol and the volume of water that are needed. A plot of the partial molar volumes of EtOH and H2O is given in Problem 6.61.
Problem 6.61
Consider a binary mixture of ethanol, EtOH (CH3CH2OH) and water (H2O) . A plot of the partial molar volumes of EtOH and H2O versus mole fraction EtOH (xEtOH) is provided in the following fi gure. Note that the units of partial molar volume are on a per mass basis so that both plots fi t on the same scale. Answer the following questions: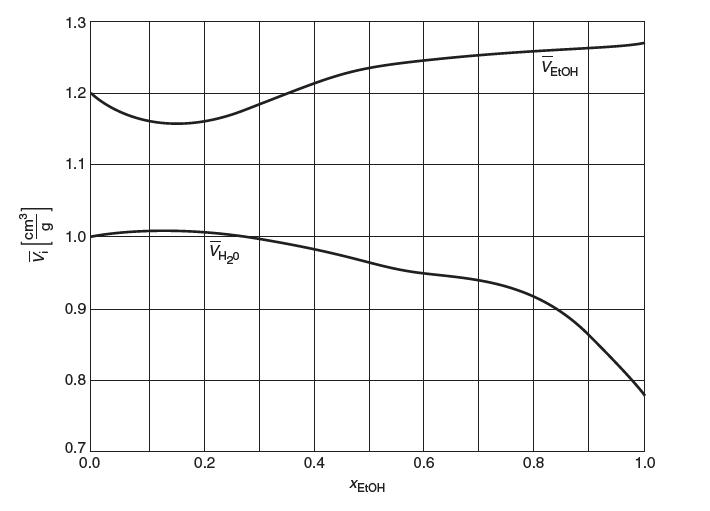
Step by Step Answer:





