Consider fi lling a gas cylinder with ethane from a high-pressure supply line. Before fi lling, the
Question:
Consider fi lling a gas cylinder with ethane from a high-pressure supply line. Before fi lling, the cylinder is empty (vacuum). The valve is then opened, exposing the tank to a 3-MPa line at 500 K until the pressure of the cylinder reaches 3 MPa. The valve is then closed. The volume of the cylinder is 50 L. For ethane, use the truncated virial equation of state, in pressure: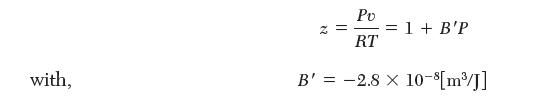
(a) What is the temperature immediately after the valve is closed?
(b) If the cylinder then sits in storage at 293 K for a long time, what is the entropy change of the universe (from the original, unfilled state)?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





