Consider the crystallization of species a. The molar Gibbs energy of pure species a vs. temperature
Question:
Consider the crystallization of species a. The molar Gibbs energy of pure species a vs. temperature at a pressure of 1 bar is shown below. Take the molar volume of species a in the liquid phase to be 20% larger than its molar volume as a solid.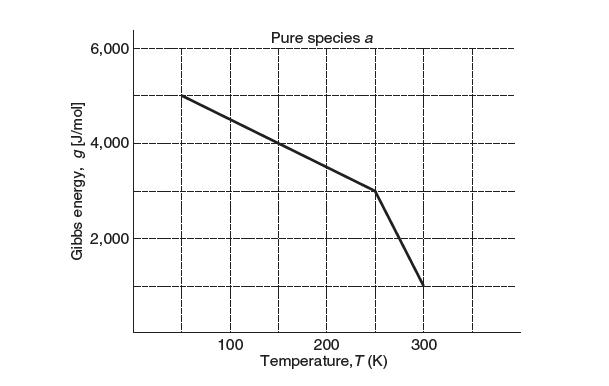
Answer the following questions:
(a) Identify the location of the freezing point on the diagram above. Identify which section of the plot corresponds to liquid and which part corresponds to solid. What is the temperature at which the liquid crystallizes? What is the Gibbs energy of the liquid at this point?
(b) Come up with a value for the entropy of the solid phase and the liquid phase.
(c) Consider this process occurring at a much higher pressure. Sketch how the plot above will change. Will the freezing point be higher or lower? Try to be as accurate as possible with the features of your sketch. Write down all your assumptions.
Step by Step Answer:





