Question: Develop a general expression for ssys for an ideal gas that goes from (v1, T1) to (v2, T2) based on the path below. S Volume
Develop a general expression for Δssys for an ideal gas that goes from (v1, T1) to (v2, T2) based on the path below.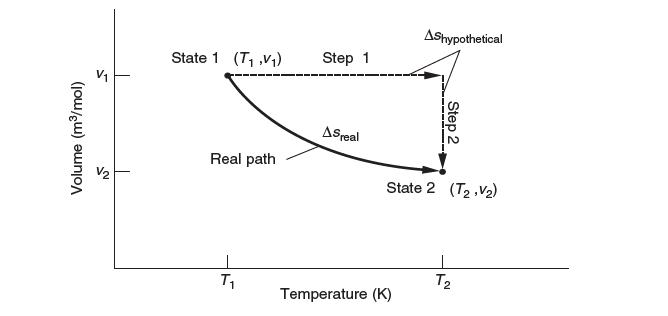
S Volume (m/mol) NT State 1 (T.V) Real path T Step 1 ASreal AShypothetical Temperature (K) Step 2 State 2 (T, V) T
Step by Step Solution
★★★★★
3.30 Rating (150 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


