Enthalpy of mixing data for binary mixtures of water (1) and acetone (2) have been fi t
Question:
Enthalpy of mixing data for binary mixtures of water (1) and acetone (2) have been fi t to the following equation: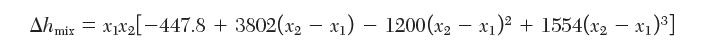
where Δhmix has units of J/mol. At 60°C, the activity coeffi cient of water in an equimolar mixture of water and acetone is 1.65. Estimate the activity coeffi cient of water in an equimolar mixture of water and acetone at 100°C. State any assumptions that you make.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





