In the two processes shown in the following fi gure, the same amount of heat, q, is
Question:
In the two processes shown in the following fi gure, the same amount of heat, q, is supplied to equal amounts (in moles) of different gases, gas A and gas B. Both gases are initially at room temperature. The heat capacity of gas A is greater than the heat capacity of gas B. These processes take place at constant volume. Which gas has the greater fi nal temperature? Explain.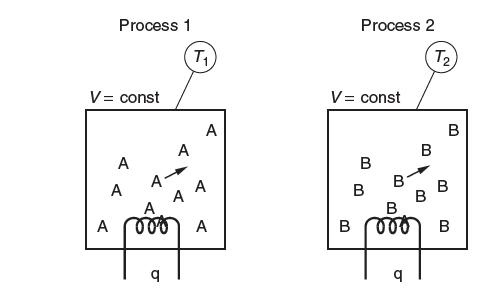
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





