Question: In this problem we seek to develop an expression for the van der Waals constants a and b in terms of molecular parameters using the
In this problem we seek to develop an expression for the van der Waals constants a and b in terms of molecular parameters using the Sutherland model for potential energy.
(a) Show that writing the van der Waals model in virial form gives an expression for the second virial coeffi cient as: B = b - a/RT . The following mathematical relation is useful: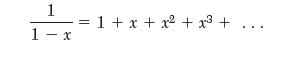
(b) Develop an expression for the second virial coeffi cient from the Sutherland model. In doing this, use the series expansion for the exponential,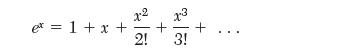
and only keep the fi rst two terms.
(c) Relate the results from parts A and B to develop an expression for the van der Waals parameters, a and b.
.. + 8 + x + x + [= 1-x 1
Step by Step Solution
3.47 Rating (154 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


