You are a process engineer in charge of growing solid silicon from a feed of SiCl4 and
Question:
You are a process engineer in charge of growing solid silicon from a feed of SiCl4 and H2 gases. The growth process can be described by the following chemical reaction: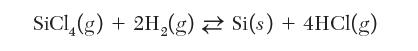
The reactor pressure is 100 Pa.
(a) Economics dictates that you need at least a 75% utilization of SiCl4; that is, 75% of the SiCl4 in the feed needs to end up as Si(s). Consider a stoichiometric feed 11 mol SiCl4 : 2 mol H2 2 . What is the minimum possible temperature in the reactor to obtain this objective. State any assumptions that you make.
(b) Your supervisor tells you that the temperature calculated in part
(a) is too high. She suggests two possible strategies for decreasing the minimum reactor temperature. Indicate the effect of each of the following process changes on the minimum possible reactor temperature to obtain a utilization of 75%. Explain your reasoning.
(i) Decrease the reactor pressure.
(ii) Dilute the feed stream to a ratio of 1 mol SiCl4: 100 mol H2.
Step by Step Answer:






