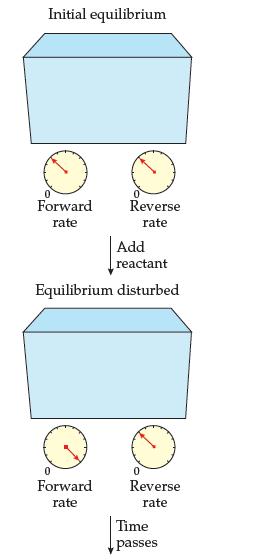
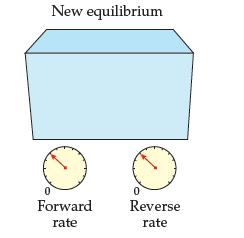
A reaction at equilibrium is disturbed as shown and then returns to equilibrium. The temperature remains constant.
Question:
A reaction at equilibrium is disturbed as shown and then returns to equilibrium. The temperature remains constant.
(a) How do the values of Keq compare for the initial and new equilibria?
(b) Something is wrong with the diagram.
What is it? How should it be corrected?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





