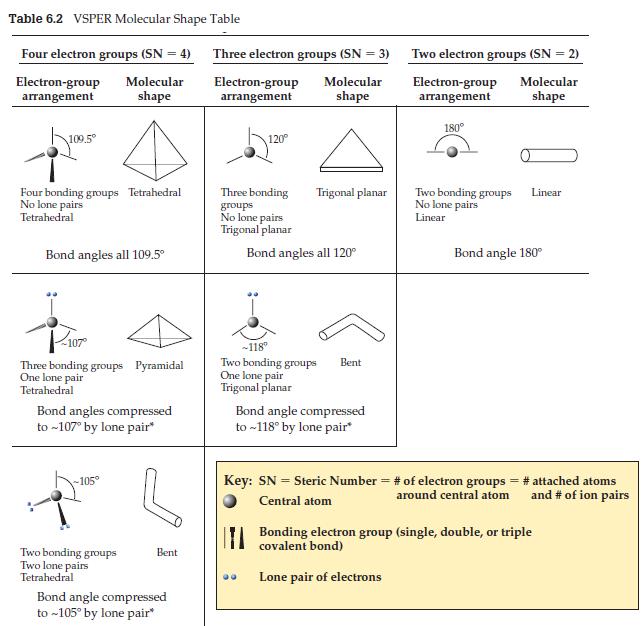
A student forgets that the N in ammonia, NH 3 , has a lone pair as well
Question:
A student forgets that the N in ammonia, NH3, has a lone pair as well as its three single bonds. After checking Table 6.2, he mistakenly draws the molecule—three bonding groups, no lone pairs— as having a trigonal planar shape. If the ammonia molecule really were trigonal planar, how would the intermolecular forces differ from what they actually are?
Transcribed Image Text:
Table 6.2 VSPER Molecular Shape Table Four electron groups (SN = 4) Molecular shape Electron-group arrangement 109.5° Four bonding groups Tetrahedral No lone pairs Tetrahedral Bond angles all 109.5⁰ Three bonding groups Pyramidal One lone pair Tetrahedral Bond angles compressed to ~107° by lone pair* -105° Two bonding groups Two lone pairs Tetrahedral Bent Bond angle compressed to ~105° by lone pair* Three electron groups (SN = 3) Molecular shape Electron-group arrangement 120° Three bonding groups No lone pairs Trigonal planar Trigonal planar Bond angles all 120° -118° Two bonding groups One lone pair Trigonal planar Bent Bond angle compressed to ~118° by lone pair* Two electron groups (SN = 2) Electron-group Molecular shape arrangement 180° Two bonding groups No lone pairs Linear Linear Bond angle 180° Key: SN = Steric Number = # of electron groups = # attached atoms Central atom around central atom and # of ion pairs Bonding electron group (single, double, or triple covalent bond) Lone pair of electrons
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Explanation If ammonia molecule were trigonal planar then it would be a nonp...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
We left three possible situations out of Table 6.2: 1. Four electron groups (SN = 4): one bonding group and three lone pairs. 2. Three electron groups (SN = 3): one bonding group and two lone pairs....
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Problem 3.3 Minimize the functional J(x()) = f sx (s) x (s) ds, = 1 sr (s) i (s) ds, 0 subject to the endpoint conditions x (0) = 0 and x (1) = 1.
-
In cost analysis, what governs which costs are to be included in the study?
-
The New Performance Studio is looking to put on a new opera. They figure that the set-up and publicity will cost $400,000. The show will go on for 3 years and bring in after-tax net cash flows of...
-
Refer to the information in Exercise 17-1. Assume that the following information is available for the companys two products for the first quarter of 2017. Required Compute activity rates for each...
-
Hampton Corporation??s balance sheet at December 31, 2011, is presented below. During 2012, the following transactions occurred.1. On January 1, 2012, Hampton issued 1,200 shares of $40 par, 7%...
-
A chimpanzee is swinging through the jungle on a 1 = 22.3 m-long vine, as shown. If the chimpanzee is to reach a tree branch h = 23.2 m above the horizontal jungle floor, what minimum speed must he...
-
Explain the difference between electron-group geometry and molecular shape. How do you use electron-group geometry when deciding what shape a molecule has?
-
Draw the three-dimensional shape of methanol, CH 3 OH. Indicate the numeric value of all bond angles. Is this molecule polar? If so, draw the molecular dipole moment vector.
-
An investment banker is analyzing two companies that specialize in the production and sale of candied apples. Old-Fashion Apples uses a labour-intensive approach, and Mech-Apple uses a mechanized...
-
Alpar Corporation formed a wholly owned subsidiary, Besub Enterprise, in country X (whose currency is the FC) on 31 December 20x0 with an initial paid-up capital of FC 10,000,000. Besub reported...
-
Explain what a functional currency is and discuss its significance.
-
Explain how financial assets and financial liabilities are classified under IFRS 9.
-
Explain why changes in the fair value of financial assets classified as carried at fair value through profit or loss differ from those classified as FVOCI.
-
How is a change in the functional currency of a foreign operation accounted for?
-
The assets and liabilities of Post Fir as of December 31, 2012, and revenues and expenses for the year ended on that date follow. Beginning retained earnings was $114,000, and dividends totaled...
-
How do the principles of (a) Physical controls and (b) Documentation controls apply to cash disbursements?
-
Identify the reagents necessary to produce each of the following compounds via an aldol reaction. (a) (b) (c) (d) (e)
-
Using formaldehyde and acetaldehyde as your only sources of carbon atoms, show how you could make each of the following compounds. You may find it helpful to review acetal formation. (a) (b) (c) (d)...
-
Draw a mechanism for the following transformation: NaOH, heat
-
How do advances in systems biology and computational modeling contribute to our understanding of the integrated network of metabolic, respiratory, and signaling pathways that govern cellular...
-
What is your investment risk appetite, more specifically, risk tolerance? (risk-averse, risk-neutral, or risk seeker) As a Consequence, which investment scenarios will be your first, second, and the...
-
Inflation is expected to be an effective 2.1% p.a. for the next five years and an effective 2.6% p.a. thereafter. R would like to make semi-annual deposits, each of the same purchasing power as...

Study smarter with the SolutionInn App


