Consider the balanced chemical equation (a) How many grams of CO could be produced from 10.0 g
Question:
Consider the balanced chemical equation
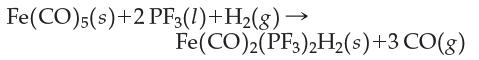
(a) How many grams of CO could be produced from 10.0 g of PF3, excess Fe(CO)5, and excess H2?
(b) How many grams of CO could be produced from 5.0 moles of Fe(CO)5, 8.0 moles of PF3, and 6.0 moles of H2?
(c) How many moles of CO could be produced from 25.0 g of Fe(CO)5, 10.0 g of PF3, and excess H2?
(d) The density of hydrogen gas at room temperature and atmospheric pressure is 0.0820 g/L. When 5.00 L of hydrogen gas at room temperature and atmospheric pressure is mixed with excess Fe(CO)5 and excess PF3, the mass of CO collected is 13.5 g. What is the theoretical yield (in grams) and the percent yield of CO?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





