Consider the decomposition reaction of ozone into oxygen: 2O 3 (g) 3O 2 (g) Suppose the
Question:
Consider the decomposition reaction of ozone into oxygen: 2O3(g) → 3O2(g) Suppose the mechanism for this reaction is just the collision between two ozone molecules, as shown in the following elementary step:
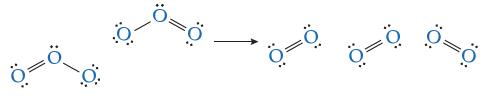
Shown here is a possible transition state. Indicate which bond(s) are breaking and which bond(s) are forming (some lone pairs of electrons are omitted to make the diagram clearer).
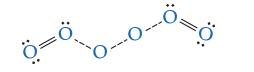
Transcribed Image Text:
11 :O: T :O: ³0: || O: :O:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Saikumar Ramagiri
Financial accounting:- Journal and ledgers, preparation of trail balance and adjusted trail balance Preparation of income statement, retained earning statement and balance sheet Banks reconciliation statements Financial statement analysis Cash flow statement analysis (both direct and indirect methods) All methods of Depreciations Management Accounting:- Ratios Budgeting control Cash budget and production budget Working capital management Receivable management Costing:- Standard and variance costing Marginal costing and decision making Cost-volume-profit analysis Inventory management (LIFO, FIFO) Preparation and estimation of cost sheet Portfolio management:- Calculation of portfolio standard deviation or risk Calculation of portfolio expected returns CAPM, Beta Financial management:- Time value of money Capital budgeting Cost of capital Leverage analysis and capital structure policies Dividend policy Bond value calculations like YTM, current yield etc International finance:- Derivatives Futures and options Swaps and forwards Business problems Finance problems Education (mention all your degrees, year awarded, Institute/University, field(s) of major): Education Qualification Board/Institution/ University Month/Year of Passing % Secured OPTIONALS/ Major ICWAI(inter) ICWAI inter Pursuing Pursuing - M.com(Finance) Osmania University June 2007 65 Finance & Taxation M B A (Finance) Osmania University Dec 2004 66 Finance & Marketing. B.Com Osmania University June 2002 72 Income Tax, Cost & Mgt, Accountancy, Auditing. Intermediate (XII) Board of Intermediate May 1999 58 Mathematics, Accountancy, Economics. S S C (X) S S C Board. May 1997 74 Mathematics, Social Studies, Science. Tutoring experience: • 10 year experience in online trouble shooting problems related to finance/accountancy. • Since 6 Years working with solution inn as a tutor, I have solved thousands of questions, quick and accuracy Skills (optional): Technical Exposure: MS Office, SQL, Tally, Wings, Focus, Programming with C Financial : Portfolio/Financial Management, Ratio Analysis, Capital Budgeting Stock Valuation & Dividend Policy, Bond Valuations Individual Skills : Proactive Nature, Self Motivative, Clear thought process, Quick problem solving skills, flexible to complex situations. Achievements : 1. I have received an Award certificate from Local Area MLA for the cause of getting 100% marks in Accountancy during my Graduation. 2. I have received a GOLD MEDAL/Scholarship from Home Minister in my MBA for being the “Top Rank student “ of management institute. 3. I received numerous complements and extra pay from various students for trouble shooting their online problems. Other interests/Hobbies (optional): ? Web Surfing ? Sports ? Watching Comics, News channels ? Miniature Collection ? Exploring hidden facts ? Solving riddles and puzzles
4.80+
391+ Reviews
552+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
The gas-phase reaction of NO with F2 to form NOF and F has an activation energy of Ea = 6.3 kJ/mol and a frequency factor of A = 6.0 108 M-1s-1. The reaction is believed to be bimolecular: (a)...
-
The decomposition reaction of N2O5 in carbon tetrachloride is 2 N2O5 -- 4 NO2 + O2. The rate law is first order in N2O5. At the rate constant is 4.82 10-3 s-1. (a) Write the rate law for the...
-
What are the five major realms of China based on climate and geography? How does the natural landscape influence the food and the culture of a nation (using China or a nation familiar to you as an...
-
9. What will the following code print on the console? int main() { } int num = 4; cout < < (num & 3 == 0 ? "first" : "second"); return 0; a. second b. first c. There is a syntax error d. It will...
-
Explain what debit and credit mean.
-
Stock X has a 10% expected return, a beta coefficient of 0.9, and a 35% standard deviation of expected returns. Stock Y has a 12.5% expected return, a beta coefficient of 1.2, and a 25% standard...
-
In LASIK surgery, a laser is used to reshape the cornea of the eye to improve vision. The laser produces extremely short \(\mathbb{N T}\) pulses of light, each containing \(1.0 \mathrm{~mJ}\) of...
-
Consider the following possible schemes for taxing a monopoly: i. A proportional tax on profits ii. A tax on each unit produced iii. A proportional tax on the gap between price and marginal cost a....
-
I just read this morning that the virus will again have an impact on us especially in some medicines due to the outbreak in China. I thought here we go again. Now the question is, was there really a...
-
Consider the basic hydrolysis (reaction with aqueous base) of (CH3) 3 CBr. The rate law is first order with respect to (CH 3 ) 3 CBr and zero order with respect to OH . What does this imply about...
-
Explain why the value of k gets larger as the temperature of a reaction mixture is increased.
-
Assume the same facts as in Problem I:8-54, except that Becky and Ken are not related and that under the terms of the loan Ken agrees to repay Becky the $5,000 plus interest (at a reasonable stated...
-
1.Nonverbal communication is difficult to interpret and complex for what 3 reasons? 2.__________ codes are a distinct, organized means of expression that consist of symbols and rules for their use....
-
Two carts were given the same amount of energy at the start with cart A being half the weight of cart, B what would the resulting speed be of the two carts?
-
What alternative course of action could the Government have taken? What measures can be taken to improve the self-regulating framework of the peg to prevent further speculation against the Hong Kong...
-
Calculate the heat transferred (In btu) through a cooling coil flowing 350GPM water with an entering temp of 43 F and a T of 11F.
-
Suppose you doubled the charge at some position in space. What would happen to its electric potential energy (assuming that it was not zero to start with)?
-
In 2005, Awnings, Inc., issues $200,000 of 15%, 20-year bonds payable at par. During 2011, when Awnings bonds are trading at 93, the company purchases and retires $100,000 par value of the bonds....
-
4. Jobe dy -Y 2 et by
-
Two balls of the same diameter are dropped simultaneously from a very tall bridge. One ball is solid lead, and the other is hollow plastic and has a much smaller mass than the solid lead ball. Use a...
-
Consider a sprinter who starts at rest, accelerates to a maximum speed v max , and then slows to a stop after crossing the finish line. Draw qualitative plots of the acceleration, velocity, and...
-
The bacterium in Figure 3.30 experiences a force due to drag from the surrounding fluid. Does the reaction force to F drag act on (a) The flagellum, (b) The body of the bacterium, or (c) The fluid?...
-
Jamie and Ross decided to conduct a checkup on their homeowners insurance policy. They noticed that they had omitted covering Jamie Lees diamond wedding band set from their policy. What if it got...
-
Medicare and Medicaid are federal programs that cover a large portion of the US population with healthcare. The two have Similar Objectives in the area of providing care, but there are significant...
-
Although Mr. Joness salary was not big enough to buy insurance for all possible risks, what protection do you think he should have had at this time?

Study smarter with the SolutionInn App


