Question: Consider the following process (e represents an electron): (a) Give the full symbol for the starting atom. (b) Give the full symbol for the
Consider the following process (e– represents an electron):
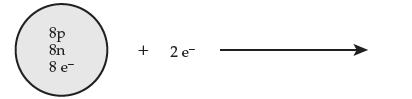
(a) Give the full symbol for the starting atom.
(b) Give the full symbol for the resulting ion.
(c) Is the product a cation or an anion? Explain.
8p 8n 8 e + 2 e
Step by Step Solution
3.56 Rating (163 Votes )
There are 3 Steps involved in it
a O b 10 c o Explanation a O indicates a neutral oxygen becuase it has 8 electrons 8 prot... View full answer

Get step-by-step solutions from verified subject matter experts


