Consider this reaction-energy profile for the reaction A B: (a) Is this reaction exothermic or endothermic?
Question:
Consider this reaction-energy profile for the reaction A → B:
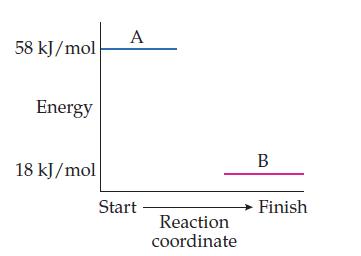
(a) Is this reaction exothermic or endothermic?
(b) Will the container in which this reaction is carried out feel hot or cold to the touch? Explain.
(c) What is the value of ΔErxn (include the sign)?
(d) What does the sign of ΔErxn tell you about this reaction?
(e) Which contain more energy, the reactants or the products?
(f) Does this reaction go uphill or downhill in energy?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





