Examine the plot of binding energy per mole of nucleons versus number of nucleons that appears on
Question:
Examine the plot of binding energy per mole of nucleons versus number of nucleons that appears on page 658. Where does your answer to Problem 16.30 put 146C relative to 126C and 136C? What does this imply about the stability of the 146C nucleus relative to that of the lighter isotopes?
Data from Page 658
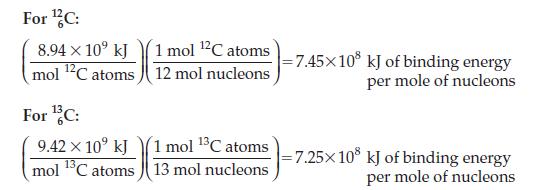
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:




