For each part of this question, choose two different species from the list below, and illustrate the
Question:
For each part of this question, choose two different species from the list below, and illustrate the indicated intermolecular interaction with a dotted line. Be sure you show all charges involved in each interaction. (Use +, -, δ+, δ-, δδ+, and δδ- where appropriate.)
If it is not possible to illustrate the specified interaction using these species, write NA (not applicable) for the answer. (Note: You may use a species from the list more than once as long as you use different species in each part).
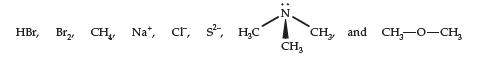
(a) An ion—dipole interaction
(b) A dipole—induced dipole interaction
(c) A dipole—dipole interaction
(d) London forces
(e) Hydrogen bonding
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





