For each reaction, write the K eq expression. Then decide which of the following equilibrium constants goes
Question:
For each reaction, write the Keq expression.
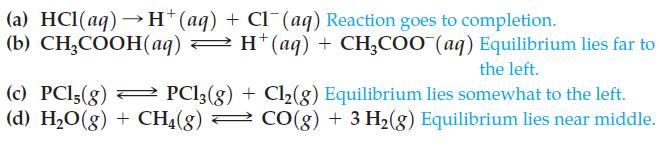
Then decide which of the following equilibrium constants goes with each reaction:
![]()
Transcribed Image Text:
(a) HCl(aq)→ H+(aq) + Cl¯(aq) Reaction goes to completion. (b) CH₂COOH(aq) → H* (aq) + CH₂COO (aq) Equilibrium lies far to the left. = (c) PC15(g) PC13(g) + Cl₂(g) Equilibrium lies somewhat to the left. (d) H₂O(g) + CH4(g) → CO(g) + 3 H₂(g) Equilibrium lies near middle.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a b c...View the full answer

Answered By

Anthony Ngatia
I have three academic degrees i.e bachelors degree in Education(English & Literature),bachelors degree in business administration(entrepreneurship option),and masters degree in business administration(strategic management) in addition to a diploma in business management.I have spent much of my life in the academia where I have taught at high school,middle level colleges level and at university level.I have been an active academic essays writer since 2011 where I have worked with some of the most reputable essay companies based in Europe and in the US.I have over the years perfected my academic writing skills as a result of tackling numerous different assignments.I do not plagiarize and I maintain competitive quality in all the assignments that I handle.I am driven by strong work ethics and a firm conviction that I should "Do Unto others as I would Like them to do to me".
4.80+
76+ Reviews
152+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
The solution containing no added KNO3 for Figure 7-1 contains 5.0 mM Fe(NO3)3, 5.0 M NaSCN, and 15 mM HNO3. We will use Davies activity coefficients to find the concentrations of all species in the...
-
1. Explain the use of strategic option in valuation. Explain how strategic options are often abused in valuation. 2. Explain the APV approach and how it is used in enterprise valuation. 3. Explain...
-
The following equilibrium constants were determined at 1123 K: Write the equilibrium constant expression KP, and calculate the equilibrium constant at 1123 K for C(s) + CO2(g)--2CO(g) CO(g) + Cl2(g)...
-
Which one of the following statements related to investigations of workplace harassment complaints under Ontario's OHSA is true? a) As a rule, such investigations should be completed with 120 days of...
-
Preparing the Investing and Financing Sections of the Statement of Cash Flows Starwood Hotels & Resorts Worldwide, Inc., is one of the worlds largest hotel and leisure companies. It conducts business...
-
Determine the moments of inertia and the product of inertia of the beam?s cross-sectional area with respect to the u and v axes. 1.5 in. 1.5 in 3 in. 30 3 in.
-
Based on the model of nuclear energy levels and transitions you have seen, would you expect 13 C to be stable?
-
Dr. Zhivgo Diagnostics Corp. income statements for 2013 are as follows: Sales....................................................................................... $2,790,000 Cost of goods...
-
At inception, a $500,000 client portfolio was invested as follows: 10% cash; 40% bonds; 50% stocks. At year-end, the portfolio held $100,000 in cash; $300,000 in equities; and $200,000 in bonds. What...
-
The equilibrium constant expression K eq for chemical reaction can be written as k f /k r because: (a) The ks are constants. (b) At equilibrium, k f [reactants] = k r [products], (the forward and...
-
Write the equilibrium constant expression for the reaction H 2 (g) + I 2 (g) 2HI(g)
-
Donald Corporation purchased a new piece of equipment to be used in its new facility. The $450,000 piece of equipment was purchased with a $50,000 down payment and with cash received through the...
-
14. What are the four basic differences between TCP and UDP TCP UDP
-
District Attorneys have decided not to prosecute drug and property crimes. Do you believe this policy is good or bad for criminal justice agencies? Please explain in details.
-
Why do we need regulation? What goals does the regulation aim to achieve? Texas has a deregulated electricity market. When the demand for electricity far exceeds the supply during the power outage,...
-
Can you describe the light OS and tiny OS, like what is there functions and purpose and how they are useful. And what is a goal it can achieve and what type of project could be done with it. (Give a...
-
The action of the British government during the Opium War is an example of unilateral political action. How can you apply the Realistic Analytical perspective to explain why the drug lords of Central...
-
What is the difference in the tax treatment of a medical insurance plan that is purchased from a third party insurer and a self-insured medical reimbursement plan?
-
1) Predict the organicproduct formed when BzCl reacts with cyclohexanol. BzCl = benzoylchloride. 2) Provide the majororganic product of the reaction below. 3) Draw the structureof the product formed...
-
A proposed design for a part of a seawall consists of a rectangular solid weighing 3840 lb with dimensions of 8.00 ft by 4.00 ft by 2.00 ft. The 8.00-ft side is to be vertical. Will this object float...
-
A platform is being designed to support some water pollution testing equipment. As shown in Fig. 5.31, its base is 36.00 in wide, 48.00 in long, and 12.00 in high. The entire system weighs 130 lb,...
-
A block of wood with a specific weight of 32 lb/ft 3 is 6 by 6 by 12 in. If it is placed in oil (sg = 0.90) with the 6 by 12-in surface parallel to the surface of the oil, would it be stable?
-
4. Find the cord tension on this beam 30.0 45.0 beam = 441 N; 6.00m long 2205 N
-
A parallel-plate capacitor has a capacitance of 1.8 F with air between the plates. The capacitor is connected to a 9.1-volt battery and charged. The battery is then removed. When a dielectric is...
-
5. Find the tension in the cable and the force exerted by the hinge on the beam. The beam has mass 50.0 kg and length 7.00m. (754N; 492N at 10.2 below hzntl) 80.0 30.0 7.00 m 2.00 m

Study smarter with the SolutionInn App


