Question: In which direction does the reaction shift when: (a) (CO 2 ) is increased? (b) The volume of the reaction vessel is decreased? (c) Ca
In which direction does the reaction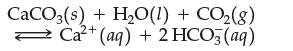 shift when:
shift when:
(a) (CO2) is increased?
(b) The volume of the reaction vessel is decreased?
(c) Ca2+ (aq) is added?
(d) CaCO3(s) is removed?
CaCO3(s) + HO(1) + CO(8) Ca+ (aq) + 2 HCO3(aq) 2+
Step by Step Solution
★★★★★
3.47 Rating (173 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

a When CO2 is increased according to Le Chateliers principle the reaction will shift to the left to ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


