Shown below are the structures for two isomers of hydroxy-benzaldehyde. Structure (a) is 2-hydroxy-benzaldehyde and structure (b)
Question:
Shown below are the structures for two isomers of hydroxy-benzaldehyde. Structure (a) is 2-hydroxy-benzaldehyde and structure (b) is 4-hydroxy-benzaldehyde. The melting point for isomer (a), 2 °C, is significantly lower than the melting point of isomer (b), 118 °C.
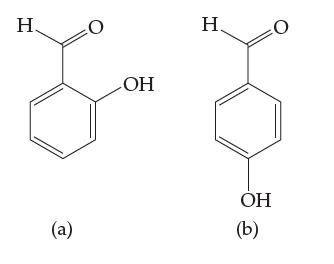
Based on your knowledge of intermolecular forces, suggest a substantive reason for this difference in melting points.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





