The diagram below shows how bent water molecules in solid ice orient themselves with respect to each
Question:
The diagram below shows how bent water molecules in solid ice orient themselves with respect to each other. Notice the large openings in the lattice of water molecules.
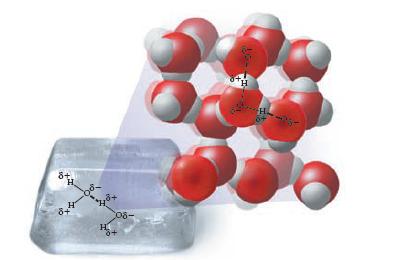
(a) Discuss why it takes energy to melt ice, and then write a statement that tells what the energy is being used for.
(b) Solid ice floats on top of liquid water. In other words, solid ice is less dense than liquid water. Based on the diagram above, come up with an explanation for why this is so and what must be true about the liquid to make it more dense than the solid.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





