The emission spectrum shown below is for neon. (a) Compare it to the emission spectrum of hydrogen.
Question:
The emission spectrum shown below is for neon.
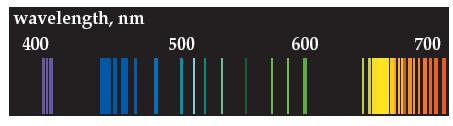
(a) Compare it to the emission spectrum of hydrogen. How is it different?
(b) Is it a continuous or discontinuous spectrum? Explain.
(c) Calculate the energy in joules of the light-blue line at 5.00 × 102 nm.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





