Two possible mechanisms for a certain reaction are: Red oxygen is the nonradioactive isotope oxygen-18, and analysis
Question:
Two possible mechanisms for a certain reaction are:
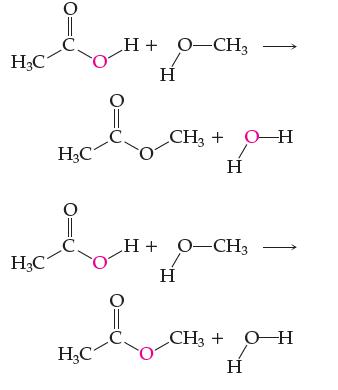
Red oxygen is the nonradioactive isotope oxygen-18, and analysis of the products reveals that all of the 18O ends up in the water.
(a) Which mechanism is correct, and which bond(s) in each reactant must be broken to form the products?
(b) If your laboratory assignment is to determine which mechanism is the right one, how would it help if 18O were radioactive?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





