Using the EMF series on page 391, decide which of the following redox reactions is spontaneous. Explain
Question:
Using the EMF series on page 391, decide which of the following redox reactions is spontaneous. Explain your answer.
(a) 3Ag + Au3+ → Au + 3 Ag+
(b) Au + 3Ag+ → 3Ag + Au3+
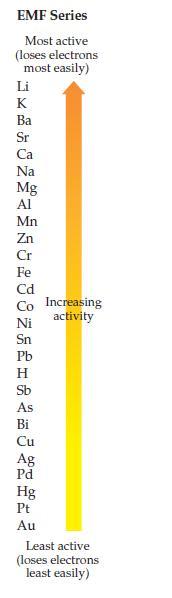
Transcribed Image Text:
EMF Series Most active (loses electrons most easily) Li HKkxQMSA MAGRGOMADHA &斑Q8HL加 Ba Sr Ca Na Mg Al Mn Zn Cr Fe Cd Co Increasing Ni Sn Pb Н Sb As Bi Cu Ag Pd Hg Pt Au activity Least active (loses electrons least easily)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a 3Ag Au3 Au 3 Ag Spontaneous Explanation The EMF series shows the standard reduction potentials of ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Using the EMF series on page 391, decide which of the following redox reactions is spontaneous. Explain your answer. (a) 3K + Al 3 + Al + 3K + (b) Al + 3K + 3 K + Al 3 + EMF Series Most active...
-
Suppose gold were not available for a wedding ring, but you still wanted a ring that would last forever and not corrode. According to the EMF series on page 391, what would be a good alternative...
-
A plumber's handbook states that you should not connect a brass pipe directly to a galvanized steel pipe because electrochemical reactions between the two metals will cause corrosion. The handbook...
-
explain the term " system development" and describe the steps involved in system development
-
In the long run, the normal selling price must be set high enough to cover what factors?
-
Tony Rondeli manufactures and sells homemade wine, and he wants to develop a standard cost per gallon. The following are required for production of a 50-gallon batch. 3,000 ounces of grape...
-
A \(4.0-\mathrm{kg}\) block and a \(2.0-\mathrm{kg}\) block are connected to opposite ends of a relaxed spring of spring constant \(300 \mathrm{~N} / \mathrm{m}\). The blocks are pushed toward each...
-
During the first month of operations, Simmons Heating and Air Conditioning, Inc., completed the following transactions: Jan 2 Simmons received $39,000 cash and issued common stock to the...
-
Let A 1 1 = 0 1 Then the 1,2 entry in the 35th power of A is (435) 12= ? Your answer should be numeric.
-
You are trapped on a desert island with plenty of water (both fresh and salt), a drinking glass, some wire, a radio, and no batteries. You do have a tin cup, a tube of toothpaste containing stannous...
-
What happens when you place a less active metal in a solution of ions of a more active metal?
-
In Exercise, determine A B. 1 A = 4 -2 B -1 3
-
5. (3 points) The following routine takes as input a list of n numbers, and returns the first value of i for which L[i]
-
In terms of brand equity, the revenue differential between a branded item such as Tropicana orange juice and a corresponding private labeled item such as Kroger orange juice is known as ____. a....
-
The current ratio is not the most stringent measure of liquidity, because it ____. Group of answer choices requires many years of past data includes some items, such as inventory, that may not be...
-
What is Linux? When would you use it? How does it differ from Windows? Are there different versions of Linux?
-
An advising or recommending effect A:He will ask his boss to sign off on his request for some vacation time B:He would release an upgrade of the software if there was more interest from customers...
-
How does the legislative grace concept help identify amounts that qualify for deduction?
-
A company has the following incomplete production budget data for the first quarter: In the previous December, ending inventory was 200 units, which was the minimum required, at 10% of projected...
-
An object is located at a distance s 0 from a spherical mirror of radius R.Show that the resulting image will be magnified by an amount R MT = 2s, + R
-
Design a little dentists mirror to be fixed at the end of a shaft for use in the mouth of some happy soul. The requirements are (1) that the image be erect as seen by the dentist and (2) that when...
-
An LED 0.60 cm tall is on the central axis 30.0 cm in front of a convex spherical mirror. If the radius of curvature of the mirror is 12.0 cm determine the location of the image, describe it, and...
-
You just deposited $9,500 in an investment account and will deposit $5000 more four years from now. What is the built-in functions on a spreadsheet to determine how much will be in the account 11...
-
Margaret had to come up with a unique data collection and analysis project to present to the class. After much hair-pulling and teeth- grinding, she came up with a cool idea. Margaret's brother mowed...
-
Given wE = 500 rpm CCW, the mechanism above: Solve using the pole-zero or the pole points method (Aronhold-Kennedy). (Length units are in inches). a. Calculate the DOF of the mechanism above b....

Study smarter with the SolutionInn App


