Question: Classify the following reactions as combination, decomposition, single-replacement, double-replacement, or neutralization: (a) Zn(s) + CuSO4(aq) (b) 2 Sr(s) + O(g) (c) Cd(HCO3)2(S) (d) HCHO(aq) +
Classify the following reactions as combination, decomposition, single-replacement, double-replacement, or neutralization:
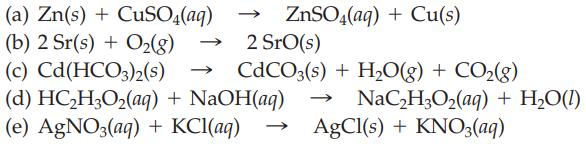
(a) Zn(s) + CuSO4(aq) (b) 2 Sr(s) + O(g) (c) Cd(HCO3)2(S) (d) HCHO(aq) + NaOH(aq) (e) AgNO3(aq) + KCl(aq) - ZnSO4(aq) + Cu(s) 2 Sro(s) CdCO3(s) + HO(g) + CO(g) >>> NaCHO(aq) + HO(1) AgCl(s) + KNO3(aq)
Step by Step Solution
★★★★★
3.39 Rating (165 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

a Singlereplaceme... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


