Which group in the periodic table has the highest ionization energy? Which group has the lowest ionization
Question:
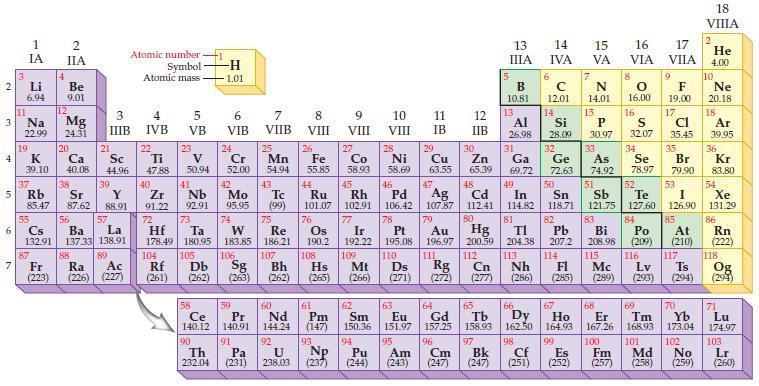
Which group in the periodic table has the highest ionization energy? Which group has the lowest ionization energy?
Periodic Table:
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 Cs 132.91 87 4 Fr (223) 2 IIA Be 9.01 12 Mg 24.31 20 Sr Y 85.47 87.62 88.91 38 Ca Sc 40.08 44.96 56 Ba 137.33 21 88 3 IIIB 39 57 La 138.91 H 89 Ac (226) (227) Ra Atomic number Symbol - Atomic mass 4 IVB 22 Ti 47.88 40 5 VB 23 V 50.94 41 Zr Nb 91.22 92.91 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 72 73 W Re Hf Ta 178.49 180.95 183.85 186.21 91 7 VIIB Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe 55.85 44 Os 190.2 108 Ru Rh 101.07 102.91 76 61 Pm (147) 9 VIII 93 27 U NP 238.03 (237) Co 58.93 Bh Hs Mt (262) (265) (266) 45 77 Ir 192.22 109 62 Sm 150.36 10 VIII 28 Ni 58.69 46 Pd 106.42 78 63 Eu 151.97 95 94 Am Pu (244) (243) 11 IB 12 IIB 96 13 IIIA 5 Cm (247) B 10.81 13 97 Bk (247) Al 26.98 14 15 IVA 6 C 12.01 14 79 81 82 Pt Au Hg TI Pb 195.08 196.97 200.59 204.38 207.2 110 111 112 113 114 Ds Rg Cn Nh Fl (271) (272) (277) (286) Si 28.09 32 17 16 VA VIA VIIA 7 N 14.01 15 P 30.97 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 16 83 Bi 208.98 115 S 32.07 34 84 Po (209) 116 F 19.00 101 17 Md (258) Cl 35.45 35 Br 79.90 53 I 126.90 64 66 70 67 68 69 Gd Но Er Tm Yb 157.25 158.93 162.50 164.93 167.26 168.93 173.04 65 Tb Dy 98 99 100 Cf Es Fm (251) (252) (257) 85 At (210) Mc (285) (289) (293) (294) Lv Ts 117 102 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The noble gases have the highest ionization ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
1. A system has the transfer function 4 0 3s +5 Obtain the total system response of the system when it is subjected to a unit ramp input. Assume system is initially at rest. Q2. A system has the...
-
A particular element has the following values for its first four ionization energies: 900, 1760, 14,850, and 21,000 kJ/mol. Without consulting a list of ionization energy values, determine what group...
-
You are working as a project manager and have identified standards and quality requirements for both the product and project. Which stage of quality management are you in? Select one: Quality...
-
If possible, completely factor the expressions in Problems 336. x - 7x + 12 X
-
Governmental Hospital. During 2011, the following selected events and transactions were recorded by Nichols County Hospital. 1. Gross charges for hospital services, all charged to accounts and notes...
-
Deedle Company purchased four convenience store buildings on January 1, 2005, for a total of $26,000,000. The buildings have been depreciated using the straight-line method with a 20-year useful life...
-
How is a common-size income statement created?
-
A company is planning to invest $100,000 (before tax) in a personnel training program. The $100,000 outlay will be charged off as an expense by the firm this year (year 0). The returns from the...
-
Given the following information answer the question. Population Companies with 500+ employees Lubbock 290,805 18 Cumulative Not available payroll of large companies Median $44,139 household income...
-
Horizontal rows in the periodic table are referred to by what two terms? Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr...
-
Based on the general trends in the periodic table, predict which element in each of the following pairs has the higher ionization energy: (a) Na or Mg (b) O or S. Periodic Table: 2 3 4 15 6 7 3 11...
-
Where do the bubbles in champagne come from?
-
As a CEO, you feel the price war in your industry is killing profits for all firms. However, you have been warned by corporate lawyers not to openly discuss pricing with rivals, whom you know...
-
Given v 1 = 5 and a = .05, find v 2 for the following F values. (a) 5.05 (b) 3.33 (c) 2.53
-
Suppose the owner in question 65 observed the sales for 60 days and found the average sales to be 115 with a standard deviation of 12. Obtain the 90 % confidence interval for the demand for bagels....
-
As a CEO, you are concerned that your firm and the industry in your country are being devastated by foreign imports. Trade lawyers suggest that you file an antidumping case against leading foreign...
-
The owner of a local bakery feels that too many bagels are thrown out every night, so he decides to estimate the demand for bagels. After a months observation, he collected 30 days sales and...
-
Given here are the data for a dependent variable, y, and independent variables. Use these data to develop a regression model to predict y. Discuss theoutput. 643105180707 X-565465746796 412951829095...
-
A heat engine has a heat input of 3 Ã 104 Btu/h and a thermal efficiency of 40 percent. Calculate the power it will produce, in hp. Source 3 x 10 Btu/h 40% HE Sink
-
Lessor Entries, Sales-Type Lease Watkins Company, a machinery dealer, leased a machine to Romero Corporation on January 1, 2011. The lease is for an 8-year period and requires equal annual payments...
-
On January 1, 2011, Palmer Company leased equipment to Woods Corporation. The following information pertains to this lease. 1. The term of the non-cancelable lease is 6 years, with no renewal option....
-
The following facts pertain to a non-cancelable lease agreement between Lennox Leasing Company and Gill Company, a lessee. The Collectibility of the lease payments is reasonably predictable, and...
-
The Easter bunny has a bag containing 23 blue Easter eggs and 23 red Easter eggs. He proposes the following game to one of his helpers, stating that the one that wins receives the big chocolate...
-
Solve the minimum-span problem for the network shown below 8 B 2 10 D E H
-
A company wants to build a new manufacturing facility. The company plans to work with five suppliers. The cost per unit distance traveled is the same for each supplier, but the number of trips per...

Study smarter with the SolutionInn App


