Consider an exothermic reaction occurring in a batch reactor, as shown in Figure 6.10. The reaction mechanism
Question:
Consider an exothermic reaction occurring in a batch reactor, as shown in Figure 6.10. The reaction mechanism is a first-order reaction, ![]() and the Arrhenius reactionrate law applies, i.e.
and the Arrhenius reactionrate law applies, i.e.![]()
The system is described by the material balance,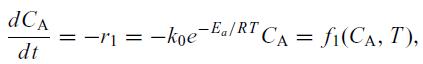
and the energy balance,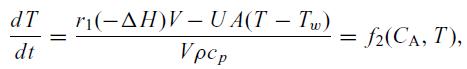
with the initial conditions CA(0) = CA0, T (0) = T0.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Mathematical Modeling In Chemical Engineering
ISBN: 9781107049697
1st Edition
Authors: Anders Rasmuson, Bengt Andersson, Louise Olsson, Ronnie Andersson
Question Posted:





