Question: The differential equation for a secondorder chemical reaction without back reaction is where c is the concentration of the single reactant and k is the
The differential equation for a secondorder chemical reaction without back reaction is
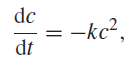
where c is the concentration of the single reactant and k is the rate constant. Set up an Excel spreadsheet to carry out Euler€™s method for this differential equation. Carry out the calculation for the initial concentration 1.000 mol lˆ’1, k = 1.000 l molˆ’1 sˆ’1 for a time of 2.000 s and for Δt = 0.100 s. Compare your result with the correct answer.
dc -ko dt
Step by Step Solution
★★★★★
3.37 Rating (172 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Here are the numbers from the spreadsheet The result of the spreadsheet calculation is ct 0... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


