Consider a fluid near its critical point, with isotherms as sketched in Figure 12.3. Assume that the
Question:
Consider a fluid near its critical point, with isotherms as sketched in Figure 12.3. Assume that the singular part of the Gibbs free energy of the fluid is of the form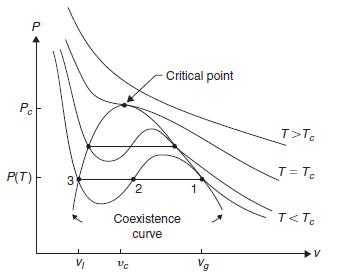 \[
\[
G^{(s)}(T, P) \sim|t|^{2-\alpha} g\left(\pi /|t|^{\Delta}\right)
\]
where \(\pi=\left(P-P_{c}\right) / P_{c}, t=\left(T-T_{c}\right) / T_{c}\) while \(g(x)\) is a universal function, with branches \(g_{+}\)for \(t>0\) and \(g_{-}\)for \(tarrow 0}=\) const.
(a) Using the above expression for \(G^{(s)}\), determine the manner in which the densities, \(ho_{l}\) and \(ho_{g}\), of the two phases approach one another as \(t \rightarrow 0\) from below.
(b) Also determine how \(\left(P-P_{c}\right)\) varies with \(\left(ho-ho_{c}\right)\) as the critical point is approached along the critical isotherm \((t=0)\).
(c) Examine as well the critical behavior of the isothermal compressibility \(\kappa_{T}\), the adiabatic compressibility \(\kappa_{S}\), the specific heats \(C_{P}\) and \(C_{V}\), the coefficient of volume expansion \(\alpha_{P}\), and the latent heat of vaporization \(l\).
Step by Step Answer:






