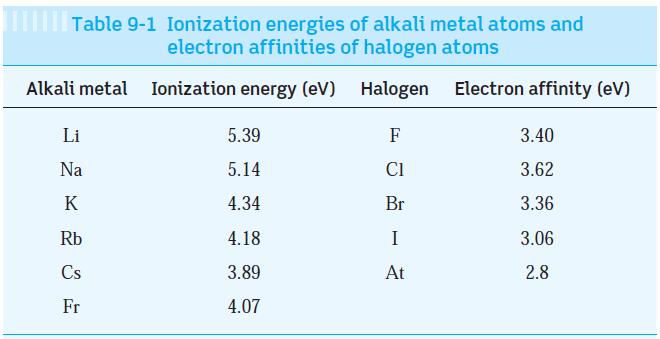
Question: Using the data in Tables 9-1 and 9-2, estimate the dissociation energy of the three ionically bonded molecules CsI, NaF, and LiI. Your results are
Using the data in Tables 9-1 and 9-2, estimate the dissociation energy of the three ionically bonded molecules CsI, NaF, and LiI. Your results are probably all higher than those in Table 9-2. Explain why.
Tables 9-1
Tables 9-2
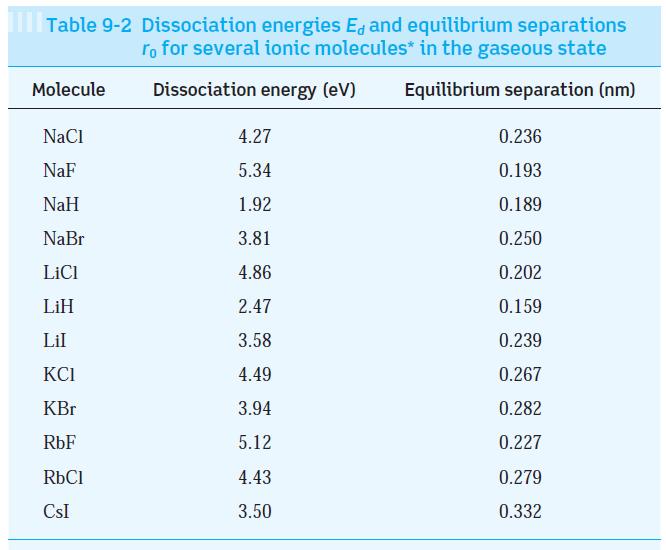
Table 9-1 Ionization energies of alkali metal atoms and electron affinities of halogen atoms Ionization energy (eV) Halogen Electron affinity (eV) Alkali metal Li Na K Rb Cs Fr 5.39 5.14 4.34 4.18 3.89 4.07 F C1 Br I At 3.40 3.62 3.36 3.06 2.8
Step by Step Solution
3.52 Rating (159 Votes )
There are 3 Steps involved in it
While E d for CsI is very close to the experimental value the ... View full answer

Get step-by-step solutions from verified subject matter experts


