Hydrides typically have rotational energies that are too widely spaced to be able to use Equation 1.99
Question:
Hydrides typically have rotational energies that are too widely spaced to be able to use Equation 1.99 at room temperature. Find zrot for HF at 300 K by summing the energies directly. Try to obtain a value that is accurate to three significant figures. Compare the numerical result to the value obtained using Equation 1.99. (Use 0.92 Â for the bond length.)
Equation 1.99
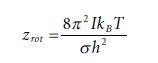
Transcribed Image Text:
Z rot 8л² Ik BT oh²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 87% (8 reviews)
To find the rotational energy of HF at 300 K by summing the energies directly we can use the following equation Erot dfrac12 h ur where Erot is the ro...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Googles ease of use and superior search results have propelled the search engine to its num- ber one status, ousting the early dominance of competitors such as WebCrawler and Infos- eek. Even later...
-
Derive a formula for the second-best relative standard to regulate a polluting monopolist.
-
Find the gravitational force between the sun and Jupiter.
-
What are the major reservoirs for the pathogen that causes legionellosis? What aspects of pathogenesis distinguish this disease from other waterborne diseases?
-
Draw an energy diagram for just the basket in Figure 9.10. Figure 9.10 Different choices of system yield different energy diagrams. (a) System basket + Earth (b) System person + rope + basket + Earth...
-
How should Intuit gauge the results of its research among younger consumers with mobile devices?
-
7. A vertical spring is fixed to one of its end and a massless plank fitted to the other end. A block is released from a height has shown. Spring is in relaxed position. Then choose the correct...
-
Use the partition functions in Equations 1.98, 1.99, and 1.100 to find the translational, rotational, and vibrational contributions to the average energy of a diatomic molecule. Compare each result...
-
The total intensity of sunlight, for all visible wavelengths striking the planet, is about 1000 W/m 2 . In a wavelength interval of about 1 nm in the visible range, the intensity is on the order of 2...
-
What is compartmentalizing and why is it necessary in fighting a fire?
-
A construction company is planning two maritime projects at different estuarine sites. Harmonic analysis of tide gauge data has identified the amplitudes (m) of four primary components, as given...
-
Show that {Vx(a), Vx } = \ x .
-
Foreign Direct Investment and Cross-Border Acquisitions Project Description: While making a decision about investing abroad a company has to consider a wide range of factors comprising the investment...
-
What organizational capabilities are required to support the launch of "Command Products"?
-
Discuss how positive psychology can be used for the greater good. Some assert that positive psychology represents the narcissistic tendencies of our culture. Explain how positive psychology can be...
-
A Harris Interactive poll conducted during January 2008 found that 944 of 1748 adult Americans 18 years or older who do not have a tattoo believe that individuals with tattoos are more rebellious....
-
What are the three kinds of research types? Explain each type.
-
Trans-2-Butene does not exhibit a signal in the double bond region of the spectrum (16001850 cm -1 ); however, IR spectroscopy is still helpful in identifying the presence of the double bond....
-
As explained previously, the concentration of an alcohol can be selected such that both a broad signal and a narrow signal appear simultaneously. In such cases, the broad signal is always to the...
-
For each of the following IR spectra, identify whether it is consistent with the structure of an alcohol, a carboxylic acid, or neither. a. b. c. d. e. f. 100- 80- 60- 40 20- 0. 2000 4000 3500 3000...
-
Daffy Duct Inc. declared a small stock dividend and issued 1,000 shares of $1 par value common stock when the price of the stock was $4 each. Identify all appropriate account titles that may be used...
-
Goody works for a firm of chartered accountants who specialise in external audit, tax advice, and retail systems. Most of your clients are small retail direct sales companies. A large national...
-
TAX 650 Milestone two Part Two: Prepare a Complex Tax Return: Complete a tax return that adheres to tax laws, regulations, and codes. Evaluate which items meet the criteria for being included in...

Study smarter with the SolutionInn App


