The water gas shift reaction (WGSR) is the reaction of carbon monoxide with steam to produce carbon
Question:
The water gas shift reaction (WGSR) is the reaction of carbon monoxide with steam to produce carbon dioxide and hydrogen and it is used in the production of hydrogen for industrial applications. 600 lbmol/hr of carbon monoxide and 30% excess steam are fed to a reactor at 600 °F and the product gases leave the reactor at temperature of 700 °F. The product gas leaving the reactor is cooled to 400 °F in a heat exchanger. The following thermodynamic data is available. Standard heats of formation (Btu/lbmol):
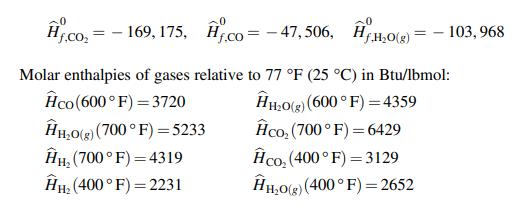
A. the standard heat of reaction for the water gas shift reaction (WSGR).
B. the molar flow rate of the product gas from the reactor in standard cubic feet per minute (SCFM).
C. the heat transfer to (or from) the reactor in Btu/hr, by using the standard heat of reaction determined earlier.
D. the heat duty of the heat exchanger in Btu/hr.
Step by Step Answer:

Chemical Engineering Principles And Applications
ISBN: 9783031278785
1st Edition
Authors: Nuggenhalli S. Nandagopal





