In general, intramolecular reactions that form rings are often disfavored entropically because it makes a flexible starting
Question:
In general, intramolecular reactions that form rings are often disfavored entropically because it makes a flexible starting material more rigid. In the case of ring-closing metathesis, however, the entropy penalty for the closure is not especially large for most systems, such as the one below used as part of a synthesis of an anti-tumor compound known as epothilone A. Why might that be?
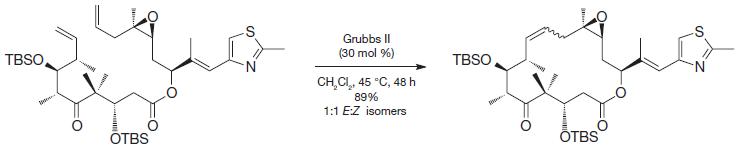
Transcribed Image Text:
TBSO THESE OTBS Grubbs II (30 mol %) CH,CI,, 45 °C, 48h 89% 1:1 E:Z isomers TBSO w OTBS N
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
It is because of two factors 1 metathesis reactions proceed via a ringopening step which is much more acidic than a ring closing step and 2 the final product is a macrocycle which will be rigid the strain energy required to form a macrocycle is higher than that of an open chain molecule In addition one must also consider the driving force for the reaction G The G term overall comes from two components the enthalpy change of the metathesis reaction ...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
A compound pendulum is defined as a rigid slab which oscillates about a fixed point O, called the center of suspension. Show that the period of oscillation of a compound pendulum is equal to the...
-
A spiro ring junction is one where two rings that share no bonds originate from a single carbon atom. Alkanes containing such a ring junction are called spiranes. (a) For the case of bicyclic...
-
Why should 3-methylcyclohexene not be used as the starting material in Problem 30b?
-
Create a T-Account Transaction Account titles Cash d Common stock Supplies Creditors accounts payable) Cash Fees earned Rent expense Cash Creditors(accounts payable) Cash Accounts receivable Fees...
-
Willie Mays, with all-around talent, was one of the greatest baseball players of all time. The numbers of stolen bases by Mays are shown in Table 77 for various years. Let n be the number of stolen...
-
Another linear operation that you are familiar with is anti-differentiation, or indefinite integration. In this problem, we will study features of antidifferentiation and relate it to...
-
Find five interesting facts in Table 1.1. TABLE 1.1 Basic Characteristics of Selected Countries GNP per capita Index of Openness Goods & Services 2009 Population Area (millions) (1,000s sq. km.) Avg....
-
(a) Analyze Facebook's financial statements and excerpts from the company's 2013 Form 10-K. Your analysis should include the preparation of common-size financial statements, key financial ratios, and...
-
Describe the relationship between average velocity of a car in motion versus the instantaneous velocity of the same car in motion. Which one matters more if you get pulled over on the freeway for...
-
Allie has bought a new apple orchard. The orchard has a single file of trees, numbered from 1 to N. Each tree has a certail number of ripe apples. Allie has a rule she wants to follow. She wants to...
-
The tranquilizing drug meprobamate (Equanil or Miltown) can be synthesized from 2-methylpentanal as follows. Give structures for meprobamate and for the intermediates AC: HCHO, HO H [A (C.H,102)]...
-
The tranquilizing drug meprobamate (Equanil or Miltown) can be synthesized from 2-methylpentanal as follows. Give structures for meprobamate and for the intermediates AC: H HCHO, HO [A (C,H,O,)]...
-
Why was it necessary to modify Beadle and Tatums one geneone enzyme concept of the gene to one geneone polypeptide?
-
Why does the body require carbohydrate? Why are wholemeal cereals nutritionally preferable to refined (white) anes? What happens if too much carbohydrate is eaten? Why do athletes eat starchy foods...
-
The bulb is a non - ohmic resistor, that is , it is not governed by the law of ohm. Doubling the potential difference at its terminals will not double the current passing through it . The light...
-
Troy Engines, Limited, manufactures a variety of engines for use in heavy equipment. The company has always produced all of the necessary parts for its engines, including all of the carburetors. An...
-
Robert Corp. granted an incentive stock option for 200 shares to Beverly, an employee, on March 14, Year 12. The option price and FMV on the date of grant was$150. Beverly exercised the option on...
-
A company purchased a highspeed industrial centrifuge at a cost of $420,000. Shipping costs totaled $12,000. Foundation work to house the centrifuge cost $8,800. An additional water line had to be...
-
Let A be a vector in Rn (written as a column), and define U = {AX | A in Mmn}. Show that U = Rm if X 0.
-
If a process has a six-sigma capability, what is the process capability index? a. 1 b. 2 c. 6 d. 12
-
Limonene, a major component of lemon oil, has the formula C10H16. (a) On reaction with excess H2 in the presence of Pt, limonene produces C10H20. What information does this provide about the...
-
Show the structures of A, B, C, and D in the following reactionsscheme: D Optically inactive H,SO. H,O B Pt C,H14 C,H12 Optically Optically inactive active 1) Hg(O,CCH3)2, H20 2) NaBH4, NAOH...
-
In Figure, suppose Br2 adds to the alkene from the bottom, rather than from the top as shown. Analyze the stereochemistry of the reaction in this case and explain which products areformed. Relative...
-
Subtract and simplify: 5x-6x+2-(-2-x+2x)
-
In order to value a company, you would need to forecast its future (a key word here) free cash flows. How would you approach this task? Using downloaded real data, try to make the forecast and see,...
-
share five (5) pieces of advice you would give the class about personal financial planning. Explain each. You might go back and look at the introductions in the first discussion for a general,...
Programming For Chemical Engineers Using C C++ And MATLAB 1st Edition - ISBN: 1934015091 - Free Book

Study smarter with the SolutionInn App


