(a) When n-heptane burns in a gasoline engine, the combustion process takes place too quickly. The explosive...
Question:
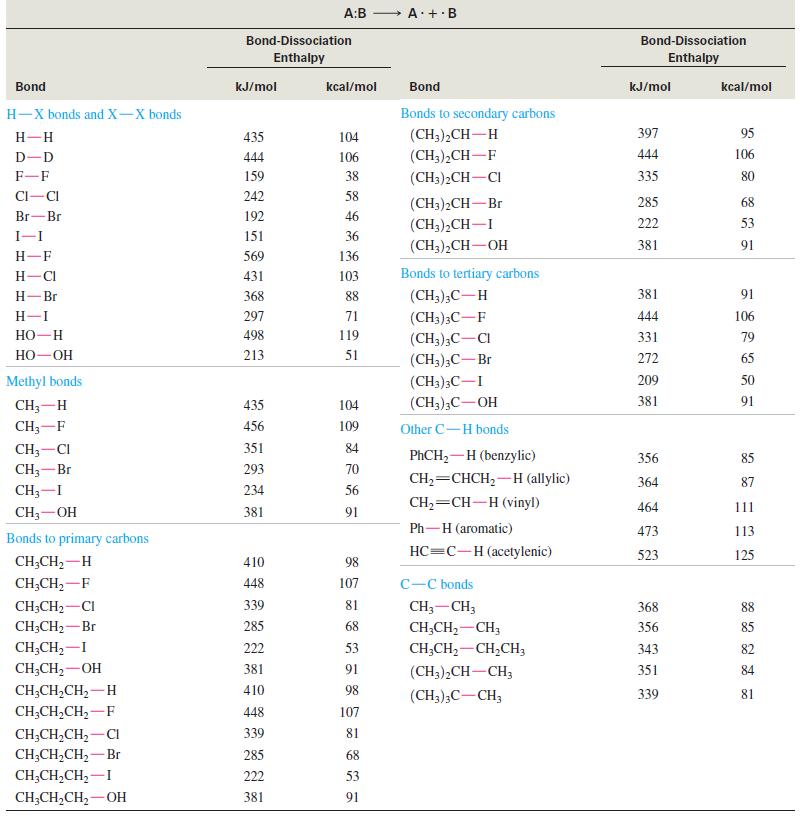
(a) When n-heptane burns in a gasoline engine, the combustion process takes place too quickly. The explosive detonation makes a noise called knocking. When 2,2,4-trimethylpentane (isooctane) is burned, combustion takes place in a slower, more controlled manner. Combustion is a free-radical chain reaction, and its rate depends on the reactivity of the free-radical intermediates. Explain why isooctane has less tendency to knock than does n-heptane.
(b) Alkoxy radicals (R-O∙) are generally more stable than alkyl (R∙) radicals. Write an equation showing an alkyl free radical (from burning gasoline) abstracting a hydrogen atom from tert butyl alcohol, (CH3)3COH. Explain why tert-butyl alcohol works as an antiknock additive for gasoline.
(c) Use the information in Table 4-2 to explain why toluene (PhCH3) has a very high octane rating of 111. Write an equation to show how toluene reacts with an alkyl free radical to give a relatively stable radical.
Table 4-2
Step by Step Answer:





